Vesber’s Foldable Capsular Buckle Structure Secures U.S. Utility Patent
Release time: Sep 15,2025

The FCB Retinal Reattachment Surgery: Redefining External Ocular Procedures
- Dramatically shorter operation time: Reduced from 50 minutes to just 10 minutes.
- Minimally invasive design: Eliminates the need for retrobulbar anesthesia, extraocular muscle traction, scleral fluid drainage, intraoperative localization, and retinal tear cryotherapy.
- Intuitive "jack-like" mechanism: Surgeons only need to implant the folded buckle onto the outer wall of the eye at the detachment site, then inflate it with normal saline. The buckle acts like a jack, gently "pushing" the detached retina back into place.
- Faster recovery & fewer complications: Significantly reduces patient discomfort, avoids a range of postoperative complications, and greatly enhances the treatment experience for patients with retinal detachment ("retinal detachment" or "RD" for short).
- Precision guided by 3D reconstruction: Uses 3D reconstruction calculations to accurately identify the location of retinal tears, effectively improving the correctness of tear reattachment and the overall reattachment rate.
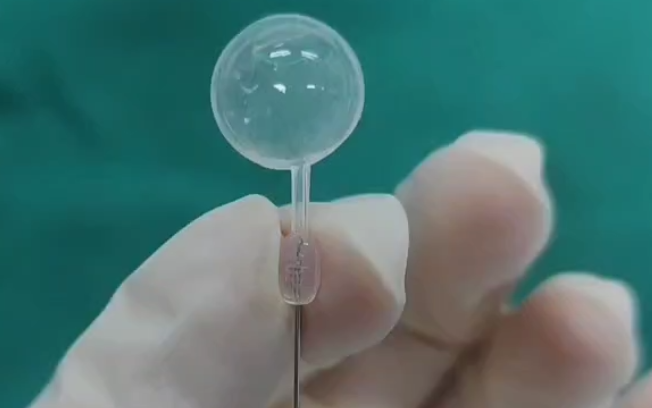
Technical Structure of the Foldable Capsular Buckle
- Buckle body: Designed to be placed within the Tenon’s capsule of the eye. It features a cavity for holding normal saline or air, with two distinct surfaces—a flat first surface and a curved second surface. The height of the buckle body along the first surface ranges from 0.2 cm to 2.0 cm.
- Self-sealing drainage component: Connected to the second surface of the buckle body and in communication with the buckle cavity, enabling controlled inflation.
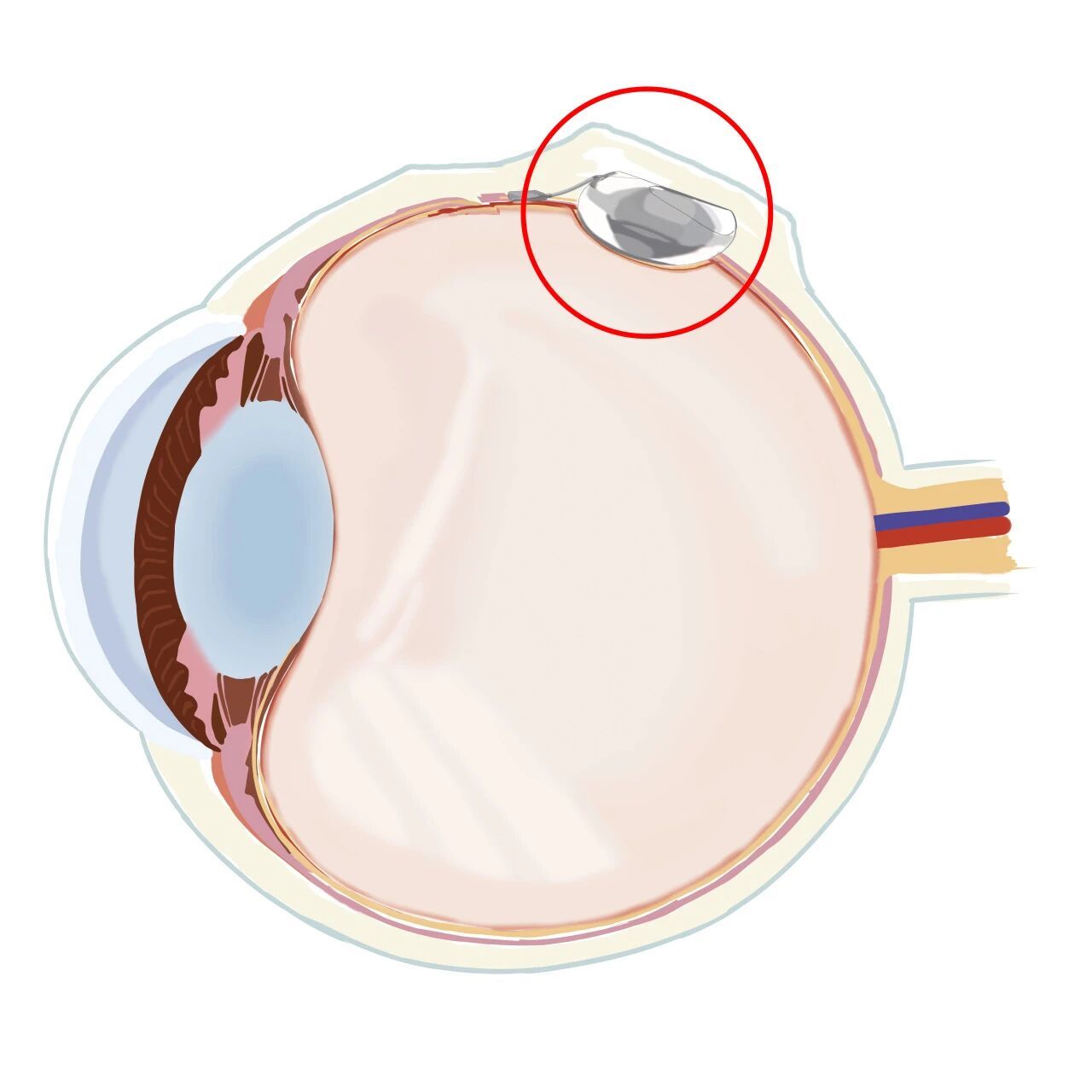
Key Advantages of the Structure
- Reduces the size of surgical incisions.
- Avoids extraocular muscle traction (no need for buckle fixation).
- Ensures no postoperative discomfort for patients.
- Simplifies surgical operation (lowers technical difficulty) and shortens overall procedure time.
Vesber’s Global Patent Portfolio: 32 Patents Across Major Markets
- 20 Invention Patents
- 11 Utility Model Patents
- 1 Design Patent
- 17 patents related to the Foldable Capsular Vitreous Body (FCVB)
- 7 patents related to the Foldable Capsular Buckle (FCB)
- 8 patents related to the posterior scleral reinforcement system for high myopia

FCVB: Vesber’s Flagship Innovation Leading Global Ocular Care
- ISO 13485 Certification (Quality Management System for Medical Devices)
- EU CE Marking
- Australia TGA Registration
- Canadian Registration Certificate (awarded on September 3, 2025)

Conclusion
The surgery not only averted the risk of eyeball atrophy but also preserved his precious visual function.
12/30
Two days later, laser photocoagulation was performed on the retinal hole in the right eye. Postoperative examination showed the retinal hole was located on the buckle ridge with good adhesion. The patient and her family were satisfied with the postoperative outcome and expressed their gratitude to Director Li's team!
12/24









