Third-Generation FCVB Approved for Registration Change by NMPA, Second-Generation FCVB Obtained UK MHRA Certificate
Release time: Apr 28,2023
Third-Generation FCVB Approved for Registration Change by NMPA, Second-Generation FCVB Obtained UK MHRA Certificate
Good News!!!
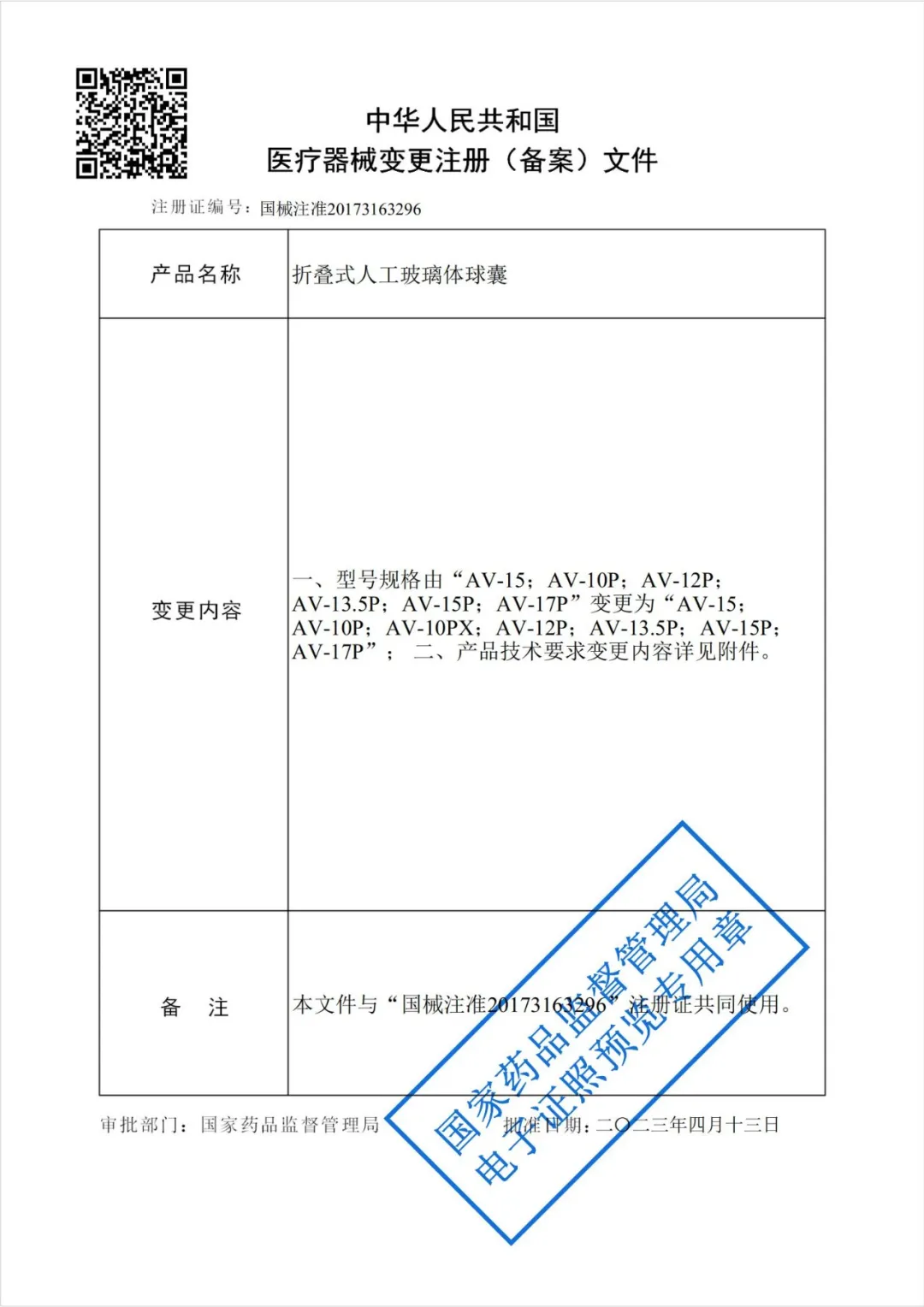
On April 13, 2023, after rigorous review by China National Medical Products Administration (NMPA), the third-generation Foldable Capsular Vitreous Body (FCVB) passed the application for change in the registration license of Class III medical devices.
In addition, the second-generation FCVB has obtained the UK MHRA certificate, marking that FCVB can be legally used in clinical practice in the UK.
1. NMPA Registration Certificate
The Foldable Capsular Vitreous Body (FCVB) is the world's first artificial organ to save eyeballs. Approved by NMPA in 2017, it obtained the registration certificate through the "green channel" for innovative medical devices. Since its launch, it has been clinically applied in over 200 hospitals across the country, helping more than 4,000 eye disease patients retain their eyeballs. It addresses the key challenges in ophthalmology of preserving eyeballs for patients with severe ocular trauma and silicone oil-dependent eyes, bringing new hope to numerous eye disease sufferers.
FCVB is not only a new product but also a new technology, embodying the wisdom of Chinese ophthalmologists. It has been continuously improved and upgraded in clinical application, evolving from the first generation to the second, and now to the third generation.
Advantages of the Third-Generation FCVB
- The drainage tube and valve are reduced in size and folded back into the eyeball;
- An additional fixation loop structure is added to secure the balloon, making it more stable inside the eye;
- The balloon diameter and optical zone diameter are fine-tuned, and a new AV-10PX model is introduced to better fit atrophic eyeballs;
- Implanted through a corneal limbus incision, with the scleral incision reduced from 4.5mm to 1.5mm.

2. UK MHRA Certificate
The full name of MHRA is "Medicines and Healthcare products Regulatory Agency". After Brexit, in accordance with the Brexit agreement, EU CE certification will gradually no longer be recognized. For medical devices, CE certification can continue to be used in the UK until June 30, 2023, but enterprises holding CE certification must have a UK Responsible Person (similar to an EU Authorized Representative) in the UK, who will register with MHRA to enter the UK GB market (England, Wales, and Scotland). From July 1, 2023, CE certification will no longer be recognized, and UKCA certification will be mandatory. The UK Medicines and Healthcare products Regulatory Agency (MHRA) is one of the two main executive agencies under the Department of Health and Social Care, and is a globally recognized authority in the regulation of medicines and medical devices, with international influence in ensuring the safety and effectiveness of medicines and promoting public health.


(△ The second-generation FCVB obtained the MHRA certificate)
FCVB obtaining the UK MHRA certificate is a high recognition by the UK Medicines and Healthcare products Regulatory Agency of the production quality system and products of Guangzhou Vesber Biotechnology Co., Ltd., laying a solid foundation for further entering the European market.
International certification is a passport for Chinese medical devices to go global. So far, FCVB has obtained multiple international certifications, including ISO13485 certification, EU CE certificate, Australian TGA certificate, as well as more than 20 patents in multiple countries. It has been clinically applied in 11 countries worldwide, including the UK, Belgium, Greece, Turkey, the Netherlands, Poland, Latvia, Georgia, Russia, and Australia.
The research and development as well as promotion of globally innovative products are full of hardships and challenges, but FCVB has also brought new hope to many eye disease patients. In the future, under the leadership of the Ocular Trauma Group and the Fundus Disease Group, Vesber will draw on the wisdom of ophthalmologists across the country to establish standardized promotion methods, so as to better serve patients. Vesber has always been committed to preserving new hope for eyeballs, writing a new chapter in eye health, striving to help eye disease patients retain their eyeballs, avoid enucleation, and "see" the future!
The surgery not only averted the risk of eyeball atrophy but also preserved his precious visual function.
12/30
Two days later, laser photocoagulation was performed on the retinal hole in the right eye. Postoperative examination showed the retinal hole was located on the buckle ridge with good adhesion. The patient and her family were satisfied with the postoperative outcome and expressed their gratitude to Director Li's team!
12/24







