Professor Yang Guang Successfully Implants FCVB in Diabetic Retinal Detachment Patient
Release time: Aug 14,2023
August 14, 2023 17:12:00. Professor Yang Guang from Chengdu Second People's Hospital successfully implanted a Folding Capsular Vitreous Body (FCVB) in a patient with bilateral diabetic retinopathy, preserving the atrophic eyeball.
August 14, 2023 17:12:00. Professor Yang Guang from Chengdu Second People's Hospital successfully implanted a Folding Capsular Vitreous Body (FCVB) in a patient with bilateral diabetic retinopathy, preserving the atrophic eyeball.
01. Case Overview
Ms. D, 49, was diagnosed with type 2 diabetes mellitus 14 years ago, essential hypertension 5 years ago, chronic kidney disease (CKD) 3 years ago, and began regular dialysis for stage 5 CKD over 2 years ago.
In recent years, she developed bilateral diabetic retinopathy. In February 2022, she underwent vitrectomy + retinal reattachment + silicone oil tamponade for right eye vitreous hemorrhage and retinal detachment. Despite three subsequent retinal reattachment surgeries, her retina remained stable. In August 2022, her left eye developed vitreous hemorrhage and retinal detachment, requiring vitrectomy + retinal reattachment + silicone oil tamponade. Nine months postoperatively, outpatient review revealed localized tractional retinal detachment in the left eye with hand motion (HM) vision at 20 cm, prompting hospital admission.
Given the complex condition, Professor Yang Guang proposed three treatment options:
- Vitrectomy + silicone oil removal + FCVB implantation
- Silicone oil removal only
- Conservative management without oil removal
Considering her monocular blindness in the left eye, risks included retinal redetachment/eyeball atrophy (if oil removed) vs. silicone emulsification/secondary glaucoma (if oil retained). After detailed consultation, FCVB implantation was selected to support the retina, prevent detachment, and preserve ocular structure.
On June 6, 2023, Professor Yang's team performed FCVB implantation. The procedure was successful, and the patient was discharged with regular follow-up.
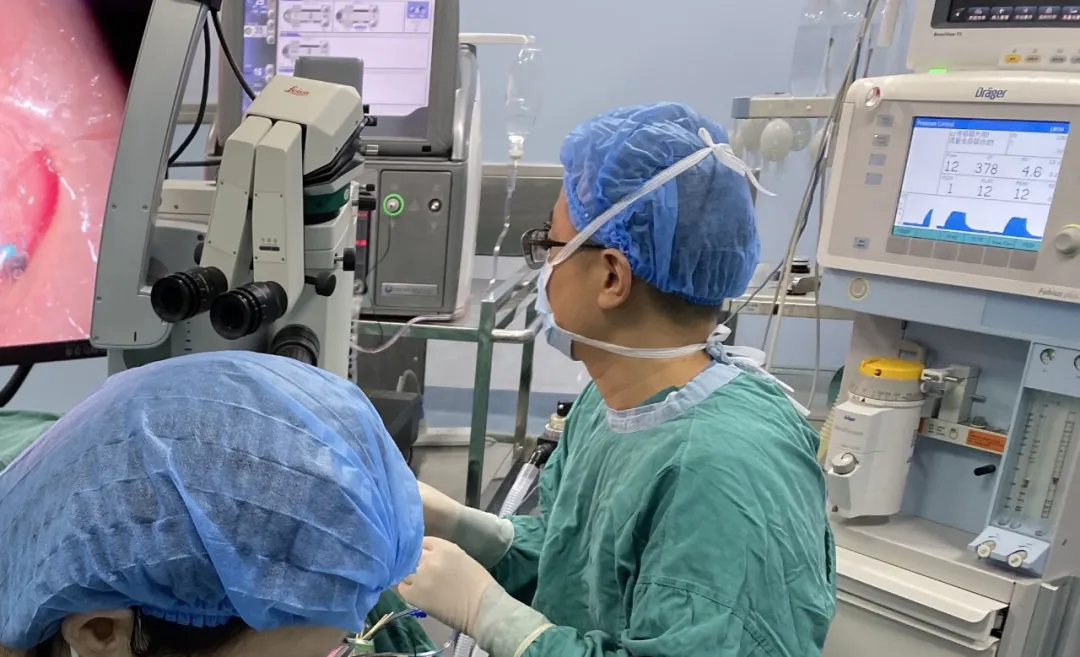
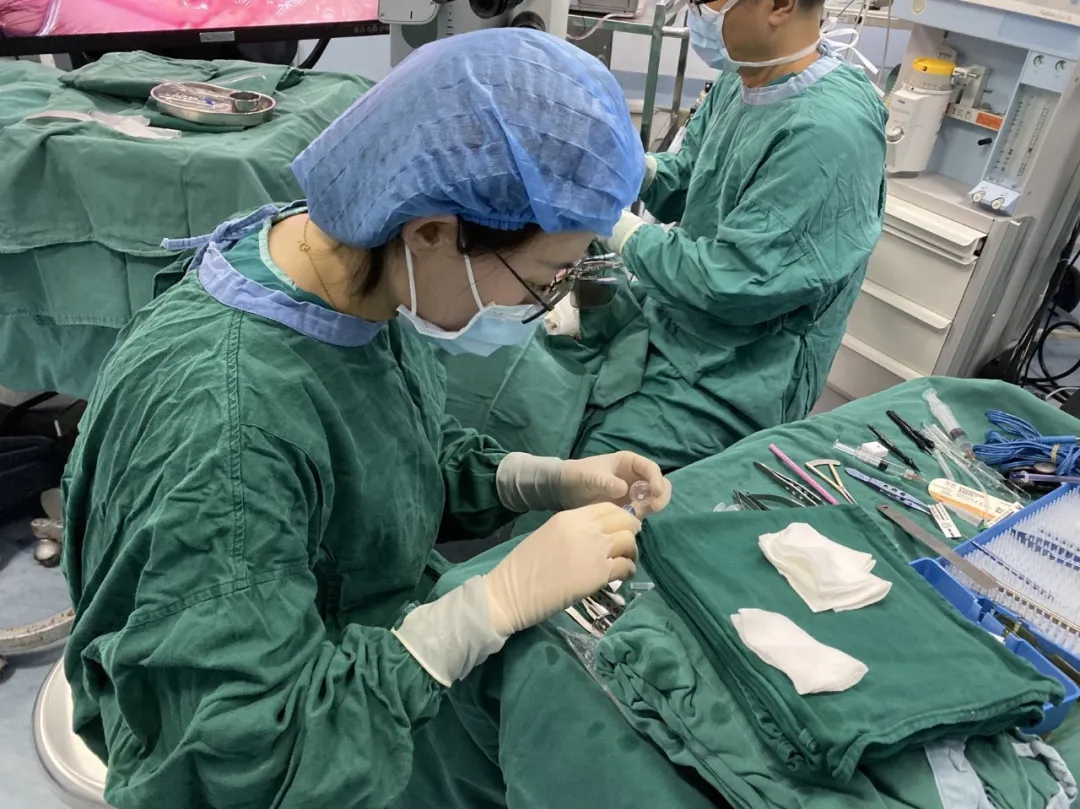
Folding Capsular Vitreous Body (FCVB)
FCVB is the world's first innovative vitreous substitute mimicking natural vitreous. Comprising a balloon, drainage tube, valve, and fixation loops, the balloon uses FDA-approved medical-grade silicone polymer. It is precisely designed via computer simulation of human vitreous cavity parameters with a crystalline plane structure. Implanted folded into the vitreous cavity, silicon oil is infused through the valve, and dual-loop fixation secures the device while isolating the ciliary body from silicone exposure.
Key advantages:
- Maintains posterior chamber space for ciliary function recovery and stable intraocular pressure (IOP)
- Prevents silicone oil emulsification and displacement
- Provides 360° retinal support and preserves ocular shape
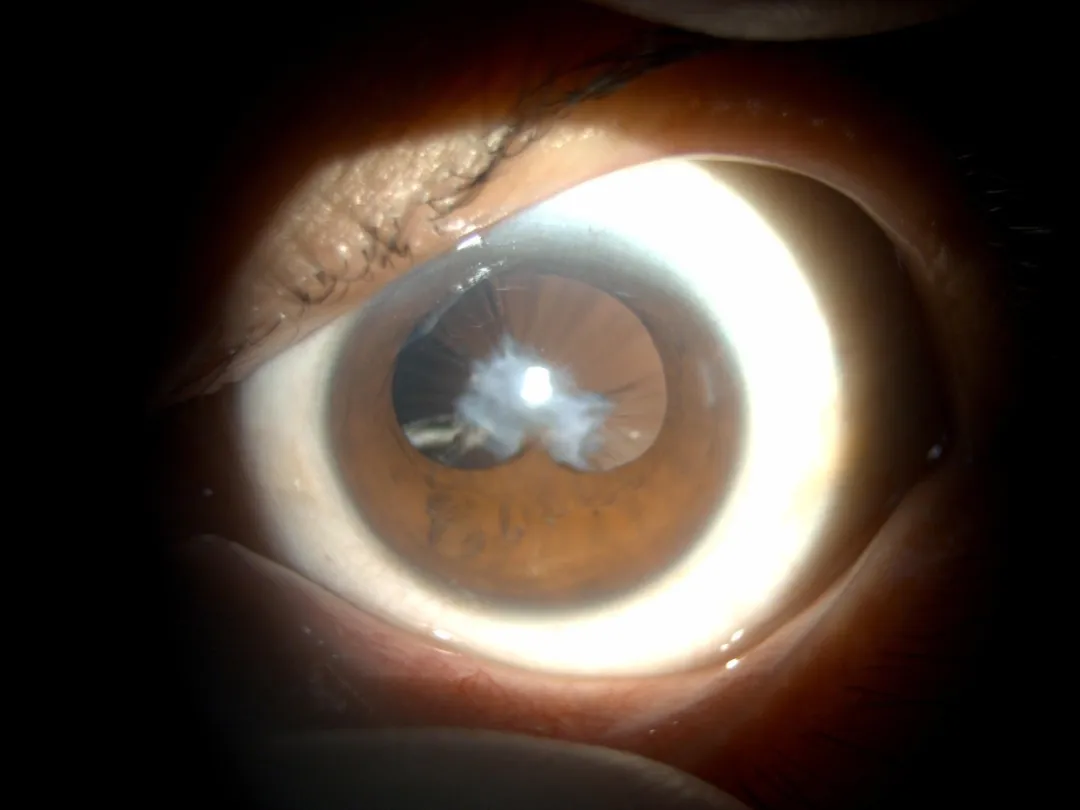
△ Preoperative Examination
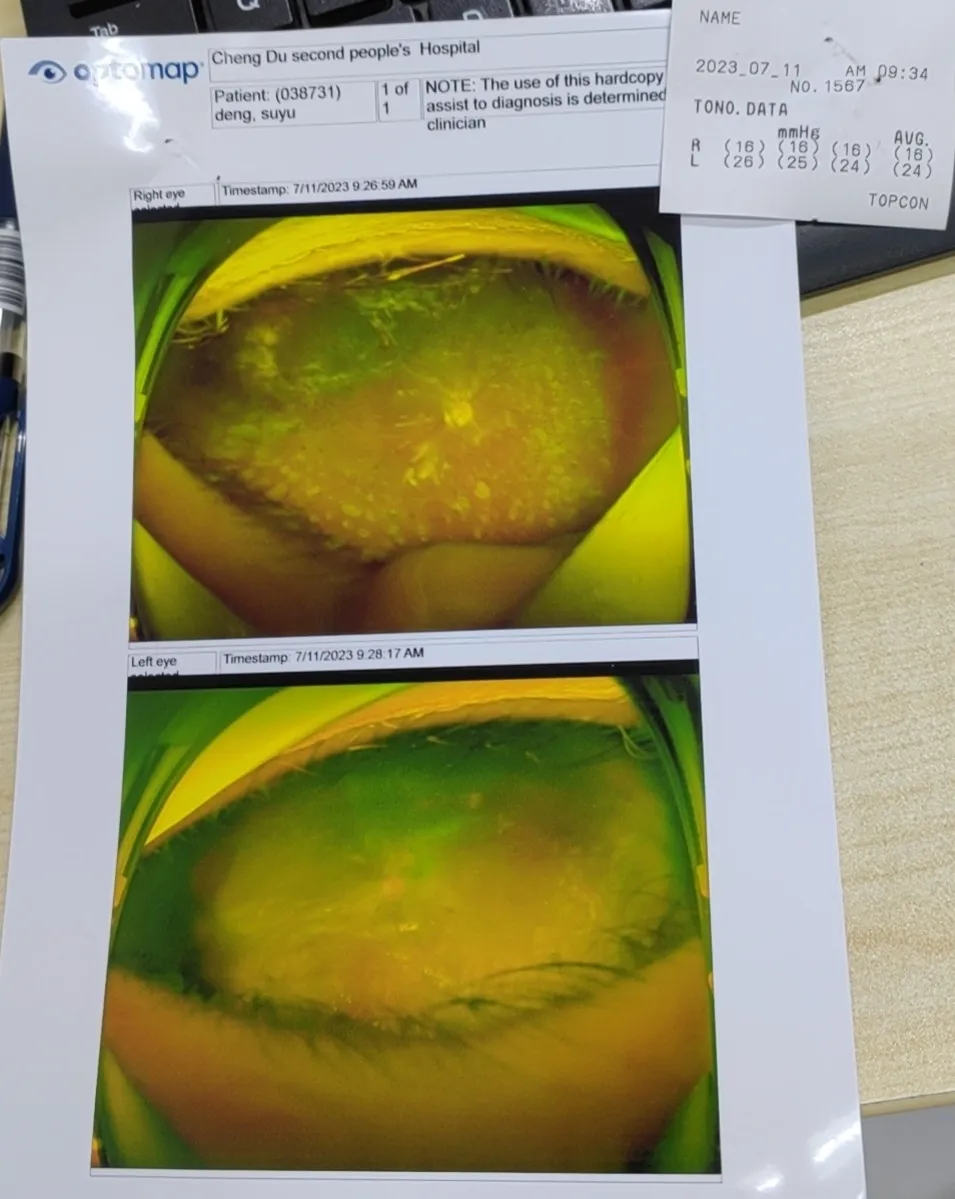
△ Postoperative Examination
Two months post-implantation, follow-up on July 24 showed:
- Left eye HM/30 cm visual acuity
- Stable IOP at 20 mmHg
- Correct FCVB positioning
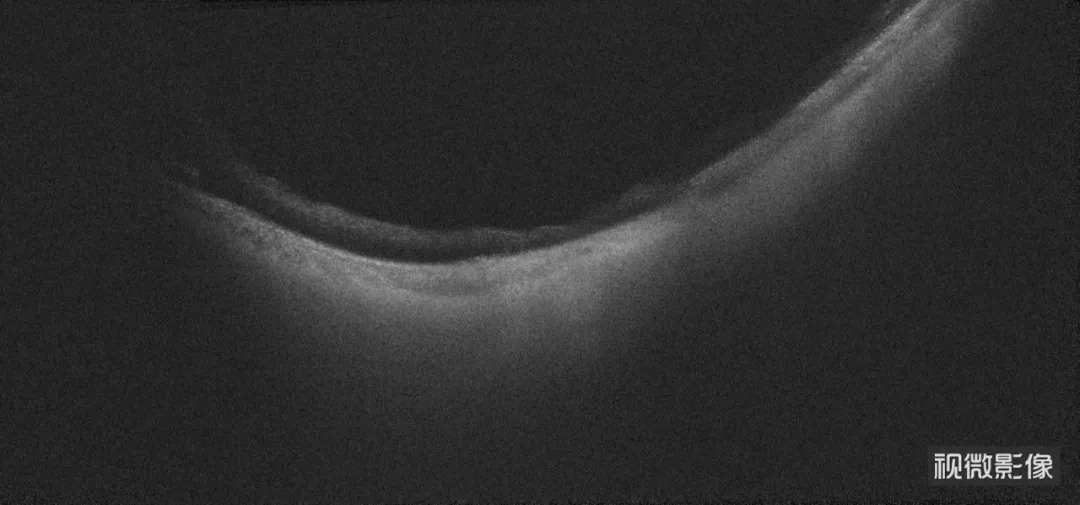
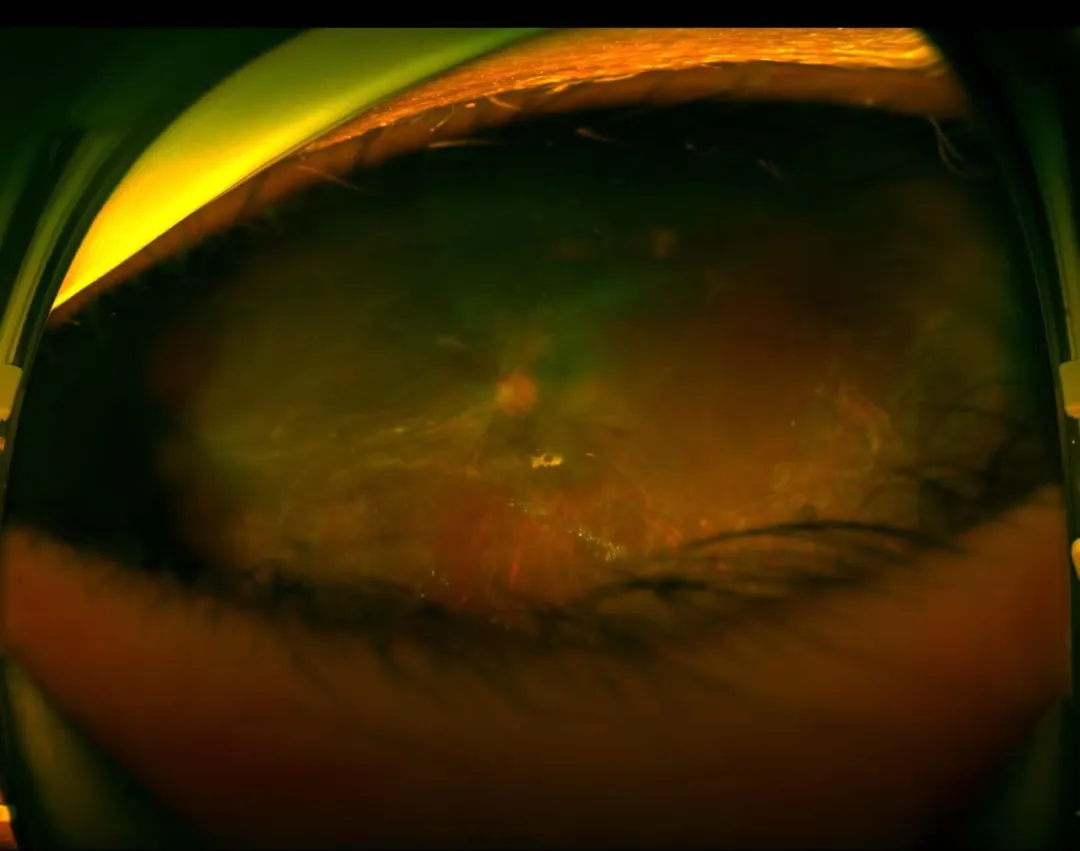
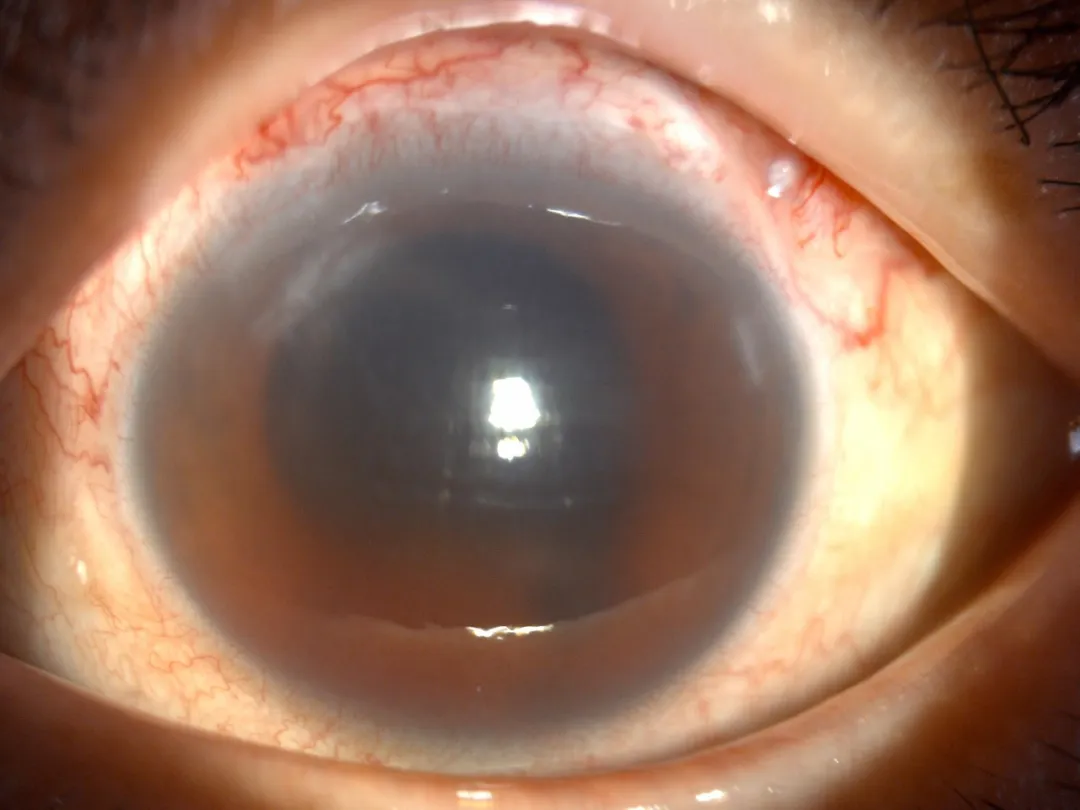
The surgery not only averted the risk of eyeball atrophy but also preserved his precious visual function.
12/30
Two days later, laser photocoagulation was performed on the retinal hole in the right eye. Postoperative examination showed the retinal hole was located on the buckle ridge with good adhesion. The patient and her family were satisfied with the postoperative outcome and expressed their gratitude to Director Li's team!
12/24







