Foldable Capsular Vitreous Body, FCVB
The product is made of US FDA approved polymer material and comprises a vitreous-like capsule, drain tube and drain valve. FCVB can accurately simulate the structure of the vitreous body of human eye. By injecting medium (silicone oil) through the drain valve, FCVB can restore the function of the vitreous body to support the retina and the eyeball, which is used to treat severe ocular trauma and silicone oil-dependent eyes.
- Product Description
-
🌍 FCVB for Severe Eye Trauma & Oil Dependency
Problems with Traditional Treatments
- 💥 Repeated vitrectomy → oil dependency
- 💥 Low pressure → phthisis bulbi
- 💥 Chronic pain → eye removal
Consequences of Eye Removal
- 💥 Permanent structural & vision loss
- 💥 Depression risk ↑63%
Solution: FCVB
Features: Capsule + tube&valve + fixation loop
- 👍 Preserves eye structure
- 👍 Restores intraocular pressure
- 👍 Prevents silicone emulsification
- 👍 Maintains natural appearance
- 👍 Treats critical conditions (rupture, long-term detachment, keratopathy, etc.)
🩺 Medical Impact
"FCVB reconstructs intraocular ecosystems, achieving triple protection: Eyeball Preservation, Ciliary Body Function Recovery, Atrophy Prevention. It restores dignity to desperate patients."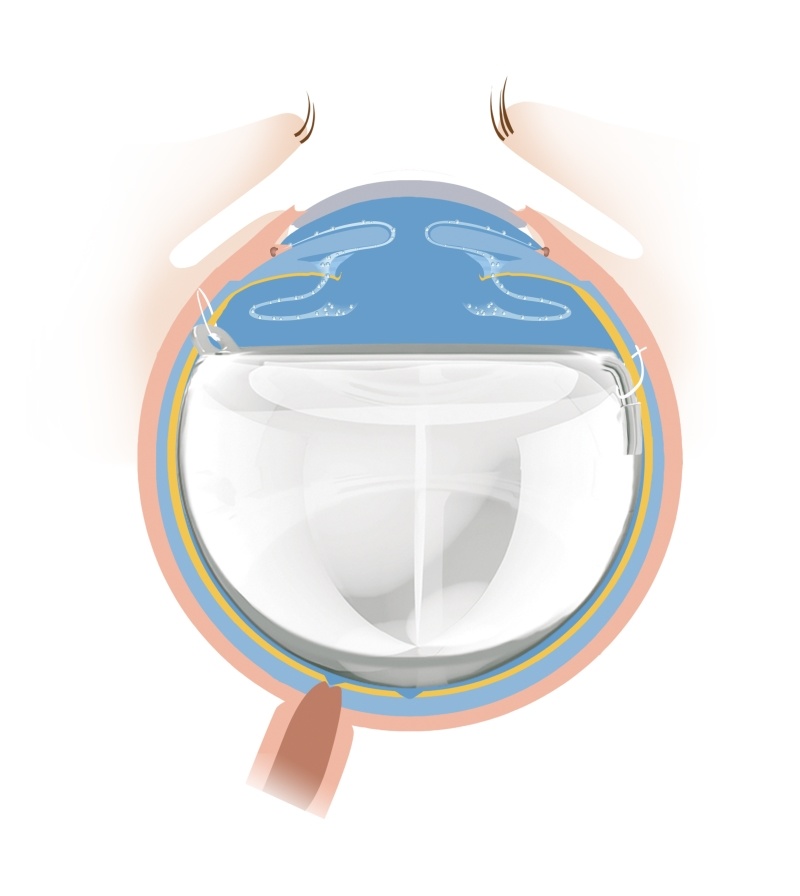
Development
The 1st generation of FCVB: It stimulates the parameters of the natural vitreous body by computer.
The 2nd generation of FCVB: It stimulates the natural vitreous body parameters by computer. The lens surface of the capsule became plane. The product retains sufficient posterior chamber space for the ciliary body to maintain its function.
The 3rd generation of FCVB
1. The drain tube and drain valve were shrunk in order to fold back into the eyeball.
2. A fixation loop was added to fix the FCVB and make the vitreous-like capsule more stable in the eye.
3. The vitreous-like capsule and the optical zone diameter were fine-tuned, and a new model, AV-10PX, was developed to make FCVB much more suitable for the atrophied eyeball.
4. The sclera incision was reduced from 4.5mm to 1.5mm, for the FCVB could be implanted at the cornea-sclera limbus.
Previous:
Related Paper
Evaluation of levofloxacin release characteristics from a human foldable capsular vitreous body in vitro
2012-01-01
Comprehensive analysis of inflammatory immune mediators of the intraocular fluid aspirated from the foldable capsular vitreous body filled-eyes
2012-01-01
Biocompatibility and retinal support of a foldable capsular vitreous body injected with saline or silicone oil implanted in rabbit eyes
2012-01-01
A novel vitreous substitute of using a foldable capsular vitreous body injected with polyvinylalcohol hydrogel
2013-01-01
Functional evaluation of a novel vitreous substitute using polyethylene glycol sols injected into a foldable capsular vitreous body
2013-01-01
A Preliminary Study to Treat Severe Endophthalmitis via a Foldable Capsular Vitreous Body with Sustained Levoflfloxacin Release in Rabbits
2013-01-01
Preliminary study on retinal vascular and oxygen-related changes after long-term silicone oil and foldable capsular vitreous body tamponade
2014-01-01
A study of the dexamethasone sodium phosphate release properties from a periocular capsular drug delivery system
2014-01-01
In vitro and in vivo release characteristics of Tacrolimus (FK506) from an episcleral drug-delivery implant
2014-01-01
FAQ
No data
Related Products
Foldable Capsular Vitreous Body, FCVB
The product is made of US FDA approved polymer material and comprises a vitreous-like capsule, drain tube and drain valve. FCVB can accurately simulate the structure of the vitreous body of human eye. By injecting medium (silicone oil) through the drain valve, FCVB can restore the function of the vitreous body to support the retina and the eyeball, which is used to treat severe ocular trauma and silicone oil-dependent eyes.
FCB is implanted through a conjunctival incision of about 4mm onto the corresponding episcleral, with saline injected via the drain valve and drain tube. After the injection of saline, the capsule can form an indentation to support the sclera and retina, allowing for the closure of retinal tears with laser treatment of rhegmatogenous retinal detachment.
It is made of medical-grade bio-compatible silicone polymer (FDA-approved material). And its major functions are encircling and buckling,it is used for encircling scleral buckling procedure.
Product Consulting
If you are interested in our products, please leave your e-mail, we will contact you as soon as possible, thank you!






